Eurofins launches COVID-19 PCR tests with at-home self-sampling options in Europe
- Like
- Digg
- Del
- Tumblr
- VKontakte
- Buffer
- Love This
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- Subscribe
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Posted: 26 January 2021 | Eurofins | No comments yet
Eurofins launches COVID-19 PCR tests with at-home self-sampling options in Europe and donates sequencing capacity to identify VUI-2020-12/01 strain reported to spread faster in the UK
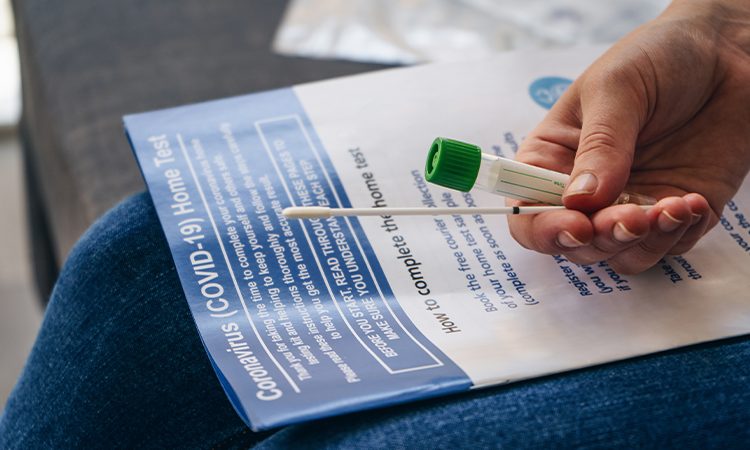

On 19 October 2020, Eurofins launched EmpowerDX SARS CoV 2 RT PCR test with at-home sampling option after it received an Emergency Use Authorization (EUA) by the FDA. Demand for the testing kit available at empowerdxlab.com is increasing significantly week-on-week.
To make patient sampling easier by non-healthcare professionals and especially for testing children, Eurofins validated a sampling method based on gargling with a sterile solution. At-home gargling self-sampling offers the advantage of a simple non-invasive sampling method, allowing patients to be tested without any constraint of medical sampling capacity.
In Germany the EmpowerDx kit for the detection of SARS-CoV-2 is now available for order at www.empowerdx.eu and via the GeLaMed app available in the Apple webstore. In Spain the kit working on saliva is available at www.empowerdx.es and www.eurofins-megalab.com/producto/test-pcr-en-saliva. The kit is delivered to the patient and collected after self-sampling, with results delivered in less than 24 hours.
As this product can significantly expand access to testing, Eurofins will apply for regulatory approval to launch similar tests in the United Kingdom, France, Belgium, Netherlands and Sweden, amongst others.
A version of this product is derived from a test developed by Eurofins Viracor in March 2020 which was ranked by the FDA as the most sensitive out of more than 115 kits evaluated with the FDA reference panel evaluation.
These tests will also detect VUI2020-12/01 virus variants recently reported to be spreading quickly in the UK. Eurofins Genomics also has significant sequencing capacity to identify this or other variants of the virus.
Given the limited understanding of the prevalence of this new variant in Europe and the consequent potential pandemic acceleration, Eurofins has decided to donate part of its sequencing capacity to national public health authorities who do not have already approved funding to identify VUI2020-12/01 in their positive samples to evaluate local prevalence of this new strain. This knowledge may be useful in deciding on local isolation and travel restrictions.